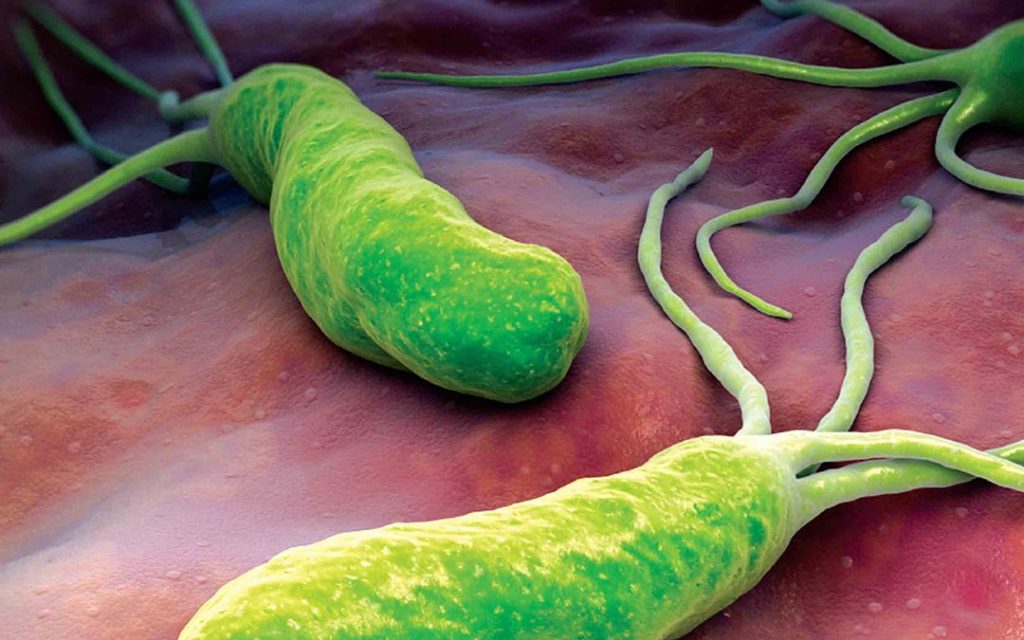
H. pylori has been identified as a group 1 carcinogen by the World Health Organization and is associated with the development of gastric cancer.
The risk of developing gastric cancer is increased by three to six times in infected persons. A meta-analysis of 51 studies revealed a decrease in mucosal inflammation and possible improvement in gastric mucosal atrophy when H. pylori is eradicated.
Treatment regimen for H pylori eradication:
- Pepto-Bismol 524 mg four times a day, plus amoxicillin 2 g four times a day, plus metronidazole
- 500 mg four times a day plus lansoprazole 60 mg once for 1 day; eradication rate 90%
Endoscopy with biopsy remains the diagnostic strategy of choice in children with persistent or severe upper abdominal pain. The goal is to detect the underlying pathophysiology and cause of symptoms not simply the presence of H pylori. The urea breath test is the noninvasive diagnostic test of choice for H pylori detection. The stool antigen test is an alternative with the monoclonal antibody based test being most reliable.
Although the use of nonsteroidal anti-inflammatory drug NSAIDS and H pylori infection are independent risk factors for peptic ulcer disease, the use of NSAIDS increases the risk of peptic ulcer disease and ulcer bleeding in patients with H pylori infection.
Original PubMed Article (Ables A. Am Fam Physician 2007; 75:351-8.)